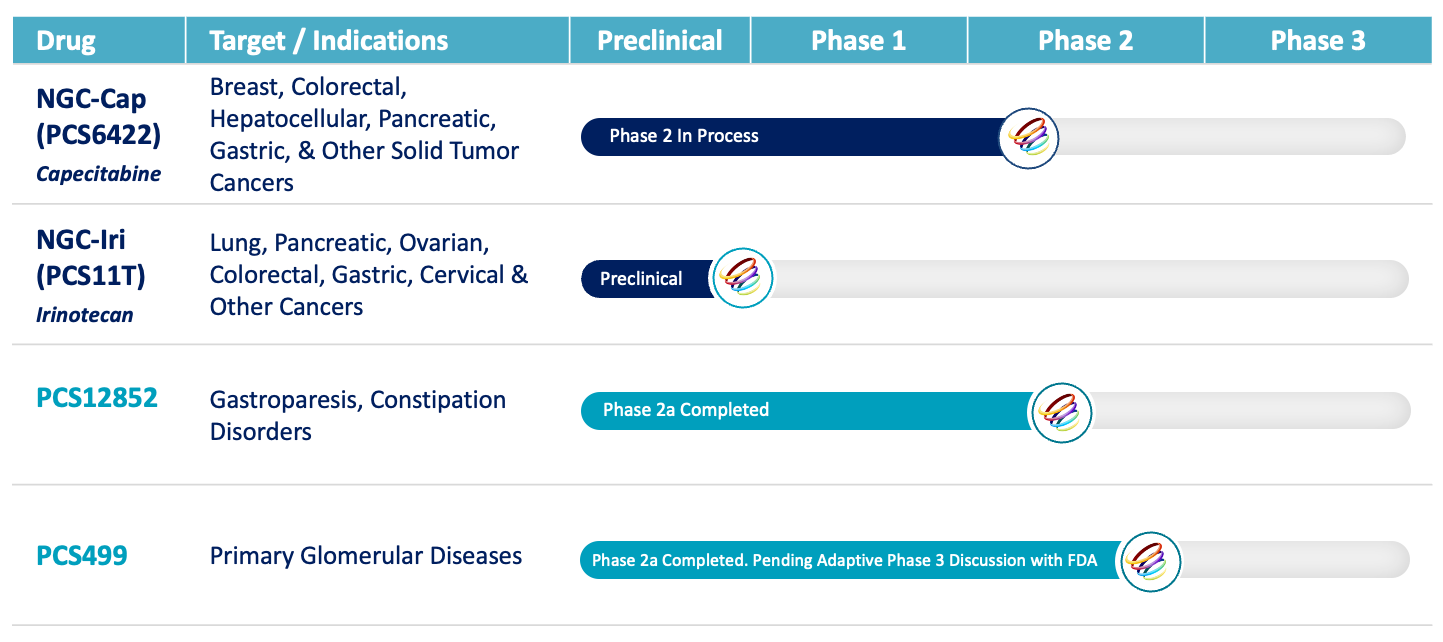
A Pipeline that is Defined by High Unmet Needs and Value
At Processa Pharmaceuticals, we focus on developing drugs that target life-threatening and life-compromising chronic diseases. These drugs are ready, or near ready, for clinical development. We acquire therapies that have demonstrated some clinical or scientific data to support the targeted treatment through studies of the drug itself, an analog of the drug, or a drug with similar pharmacological targets. We then work with the FDA to define a complete development program and out-license the drug before or after the pivotal study. Processa leverages the experience of the team to optimize trial design to create an efficient development program that decreases risk and time for regulatory review with the result of lower cost and quicker access for patients.